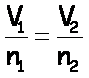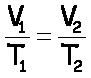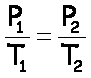“Before & After” Problems
(“Gas Laws” problems)
Because R is a constant, that means 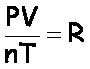 at any time. This means
at time 1 (before) and time 2 (after):
at any time. This means
at time 1 (before) and time 2 (after):
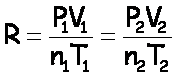
If one of these quantities is not changing, it’s the same on both sides and you can cancel it out. (If the problem doesn’t mention something, you may assume that it’s not changing.)
Sample problem:
A gas has a temperature of 25°C and a pressure of 1.5 atm. If the gas is heated to 35°C, what will the new pressure be?
Answer:
First, declare your variables. Remember to convert temperatures to Kelvin:
T1 = 25°C = 298 K T2 = 35°C = 308 K
P1 = 1.5 atm P2 = ?
Now, set up the formula, crossing out volume and number of moles, which are not mentioned:
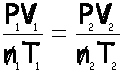

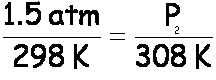

Each of the “gas laws” comes from the above, by canceling out quantities that are not changing.
|
Law |
Variables |
Formula |
|
Avogadro’s Law[*] |
n,V |
|
|
Boyle’s Law |
P,V |
|
|
Charles’s Law |
V,T |
|
|
Gay-Lussac’s Law |
P,T |
|